Specific Heat Capacity Definition
Battery capacity is defined as the total amount of electricity generated due to electrochemical reactions in the battery and is expressed in ampere hours. The value of the constant is different for different materials and depends on the process.
Does Atmospheric Pressure Affect Specific Heat Capacity Quora
In thermodynamics and solid-state physics the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat Heat capacity in a solid.
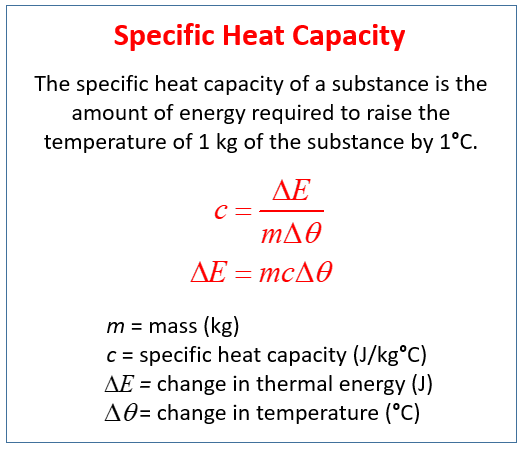
. Specific Heat Capacity c Specific heat capacity of any substance is defined as the amount of heat required to change the temperature of a unit mass of the substance by 1 degree. When latent heat is added no temperature change occurs. A specific heat capacity calculator is functioned to deliver the outcomes along with standardized units.
The Scottish scientist Joseph Black in the 18th century noticed that equal masses of. The SI unit of heat capacity is joule per kelvin JK. PediaaCom - Know about Anything.
For example the specific heat of water is 1 calorie or 4186 joules per gram per Celsius degree. For example a constant discharge current of 1 C 5 A can be drawn from a 5 Ah battery for 1 hour. Latent heat of vaporization water at 01 MPa atmospheric pressure h lg 2257 kJkg.
The Heat Capacity at Constant Pressure C p Heat capacity at Constant VolumeC v The isentropic expansion factor is another name for heat capacity ratio that is also denoted for an ideal gas by γ gamma. Elastic modulus Modulus of Elasticity 190 Gpa at 20 C. Specific Heat Capacity Unit.
Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. For example the lower specific heat capacity of fat compared to other soft tissue indicates that fat requires. Then the specific heat capacity of methanol is.
260 WmK at 20 C. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. Ahmet Aktaş Yağmur Kirçiçek in Solar Hybrid Systems 2021.
Most of the time this mould does not limit the capacity of the factory but if they receive several large orders for that specific type of product at the same time it creates a constraint. The heat Capacity formula is expressed as the product of mass specific heat and change in the temperature which is mathematically given as. 460 JkgK at 20 C 011 BtulbF at 68 F.
185 GPa at 190 C. The heat capacity of a defined object is usually expressed in joules or calories and temperature in Kelvin or Celsius. 180 GPa at 400 C.
The enthalpy of vaporization is a function of the pressure at which that transformation takes place. The below-mentioned formula can be used to calculate specific heat capacity values. Gibbs Free Energy of Formation Entropy and Heat Capacity - Definition and explanation of the terms standard.
The units of specific heat are usually calories or joules per gram per Celsius degree. The method of mixture is used almost universally by scientists as a quick easy and semi-accurate specific heat test for a solid sample but what makes this method extra special is the fact that its so simple that high school students around the world perform it as a hands-on example of how the specific heat capacities of materials are a part of the world around us. 246 x 10 3 ksi at 750 F.
Heat capacity ratio of heat absorbed by a material to the temperature change. Q mc Delta T. The heat capacity in calories per gram is called specific heat.
Heat is a transfer of energy. Dieter Haemmerich in Principles and Technologies for Electromagnetic Energy Based Therapies 2022. Therefore specific heat capacity c Qm Delta T.
If we are dealing with a gas it is most convenient to use forms of the thermodynamics equations based on the enthalpy of. Heat capacity is an extensive propertyThe corresponding intensive property is the specific heat capacity found by dividing the heat capacity of an object. C p 818 JmolK 3204 gmol 253 Jg K Converting between commonly used Units.
The definition of the calorie is based on the specific heat of water defined. Therefore the ratio between C p and C v is the specific heat. The heat capacity is a constant that tells how much heat is added per unit temperature rise.
131 Specific heat capacity. Latent heat of vaporization water at 3 MPa pressure inside a steam generator h lg 1795 kJkg. If youre given the amount of energy used the mass and initial temperature heres how to calculate the final temperature of a reaction.
For the same battery a discharge current of. 275 x 10 3 ksi at 68 F. 269 x 10 3 ksi at 375 F.
The specific heat capacity c Jkg K of tissue describes how much energy is required to change the temperature of 1 kg of tissue by 1 K 1C. Many times organizations have a seasonal CCR leading up to major cycles such as the Christmas shopping season in the United States Diwali Holidays in India Chinese New Year etc. Formula for Heat Capacity.
Definition of Specific heat capacity revealed that it is the amount of heat required to increase the temperature of 1 kilogram of any substance by 1 kelvin. Hence its derived SI unit is J kg1 K1. Gases - Specific Heats and Individual Gas Constants - Specific heat at constant volume specific heat at constant pressure specific heat ratio and individual gas constant - R - common gases as argon air ether nitrogen and many more.
Specific heat the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. It is the ratio of two specific heat capacities C p and C v is given by. Heat Capacity - The amount of heat required to change the temperature of a substance by one degree.
Heat capacity is not a state variable. It treats the vibrations of the atomic lattice heat as phonons in a box in contrast to the Einstein model which treats the solid as many individual non-interacting quantum harmonic.

Specific Heat Capacity Definition Molar Specific Heat Videos Examples
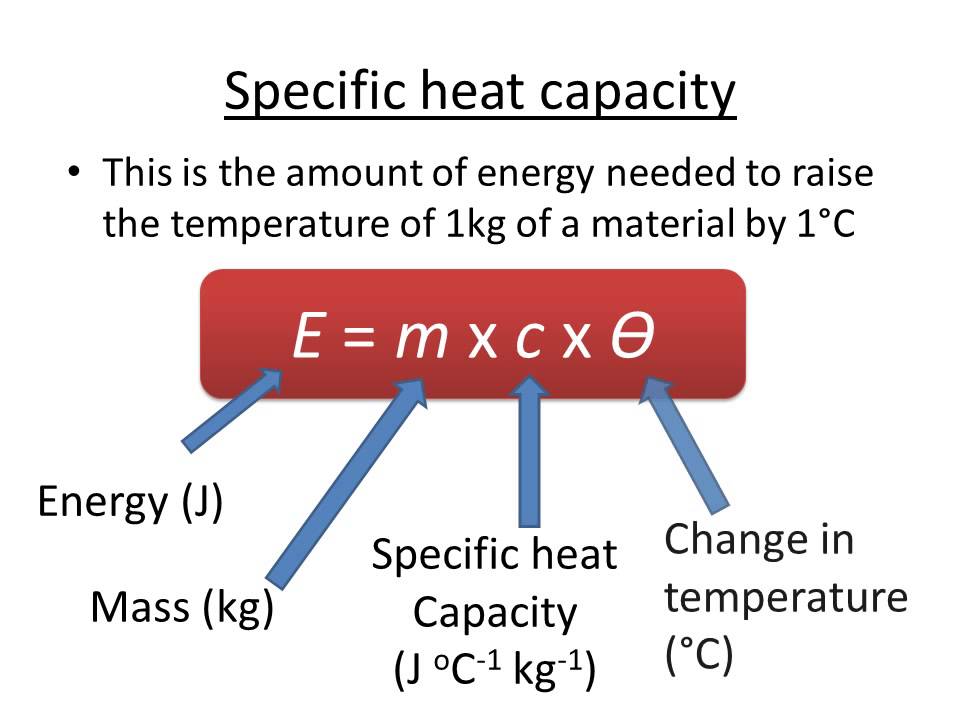
Specific Heat Capacity Mr Homer S National 4 5 Physics
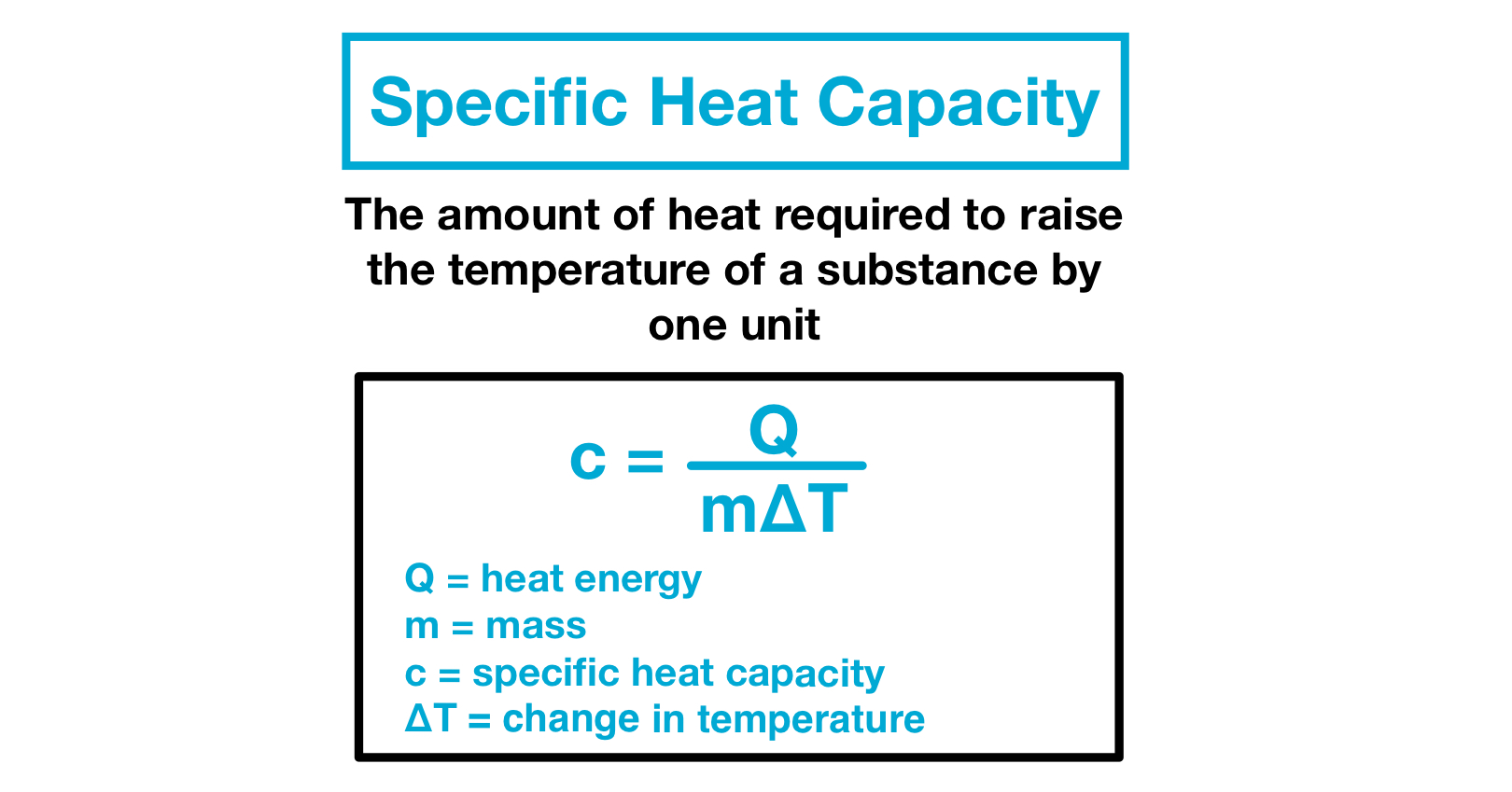
Heat Capacity Of Water Overview Importance Expii

Do You Have To Heat Formula On Sale 50 Off Www Quadrantkindercentra Nl
No comments for "Specific Heat Capacity Definition"
Post a Comment